Kirja
8. Impedance technique
8.3. Determining the impedance of an electrochemical system
We have previously stated that impedance is a frequency-dependent quantity and therefore the most convenient and reasonable way to determine it is in the frequency domain. The conversion from a time variable to a frequency variable is done using Laplace transformation, and because of this, we start with a couple of important remarks concerning Laplace transforms [1]. The Laplace transform is a linear operator:
 |
|
 |
(8.14) |
|---|
The symbol  represents the Laplace
transform, a and b are constants, and f(t) and g(t) arbitrary functions
of time;
represents the Laplace
transform, a and b are constants, and f(t) and g(t) arbitrary functions
of time;  and
and  are
transforms of f(t) and g(t). The bar over the function highlights
the frequency domain and s is the Laplace
variable (frequency).
are
transforms of f(t) and g(t). The bar over the function highlights
the frequency domain and s is the Laplace
variable (frequency).
The
transform being linear means that non-linear factors, such as product kf cs cannot be
converted (kf is a
potential dependent rate constant and cs
surface concentration of the reacting species). Non-linear factors therefore have to
be linearized near measurement potential, and because of this, the magnitude of
the input signal has to be small enough for a valid linearization. A few
examples of the most common linearizations are shown in the following
subchapters. Impedance of any given system can be determined following the
steps below:
|
1. Linearize the current-voltage equation. 2. Solve the problem in Laplace domain. 3. Remove non-periodical components of the solution with the relation
|
|---|
The
third step deserves a couple of remarks. The Laplace transform of a sinusoidal
signal takes the form  , so multiplication with a binomial
, so multiplication with a binomial  removes the denominator. All other signals
without such a denominator approach zero at the limit
removes the denominator. All other signals
without such a denominator approach zero at the limit  . Actually, there is no need to apply Equation
(8.15) in practice because all terms without
. Actually, there is no need to apply Equation
(8.15) in practice because all terms without  or
or  can be
eliminated. The following examples will clarify the method.
can be
eliminated. The following examples will clarify the method.
8.3.1 Linearization of current-voltage curves and faradaic impedance
Current-overpotential equation (6.27) was derived in Chapter 6:
 |
(6.27) |
|---|
Let us linearize this equation at the point of
equilibrium  :
:
 |
(8.16) |
|---|
 |
|
 |
(8.17) |
|---|
Equation (8.17) can be written directly in the
Laplace domain as follows:
 |
(8.18) |
|---|
Now we need to determine the surface
concentrations  and
and  from the
transfer equations, which we already did in Chapter 7. Substituting
Equations (7.7) and (7.8) to Equation (8.18) gives
from the
transfer equations, which we already did in Chapter 7. Substituting
Equations (7.7) and (7.8) to Equation (8.18) gives
 |
(8.19) |
|---|
Dividing Equation (8.19) by  and
replacing s with
and
replacing s with  , impedance can easily be found:
, impedance can easily be found:
 |
(8.20) |
|---|
Note that because i stands for current density, we needed to divide the expression by
the area A. The first term of
Equation (8.20) represents charge transfer resistance, Rct. The rate constant of the reaction, k0, can be
determined by substituting the expression for the exchange current density in
Equation (8.20). The latter term of the equation is known as the Warburg element,
which describes diffusion. Because  , it is evident
that the real and imaginary parts of the Warburg element are equal. The
faradaic impedance of the electrode reaction, Zf, is therefore
, it is evident
that the real and imaginary parts of the Warburg element are equal. The
faradaic impedance of the electrode reaction, Zf, is therefore
 |
(8.21) |
|---|
 |
(8.22) |
|---|
The characteristic feature of Warburg
impedance is an increasing straight line at an angle of 45 degrees. When
faradaic impedance and electrode capacitance are in parallel and the solution
resistance is added in front of the whole circuit, an impedance plot shown in
Figure 8.9 is obtained. The whole circuit is called the Randles circuit or Randles
equivalent circuit, shown in Figure 8.11.
Figure 8.9 shows how the values for Rs and Rct can easily be obtained. The semicircle and the straight line are usually overlapping, so only the left-hand side (high frequencies) of the semicircle is visible. Despite this, it is easy to determine the numerical values using modern software; we will come back to the fitting of circuit components later in this Chapter.
If the solution resistance and capacitance have been eliminated from the experimental data, the residual faradaic impedance can be divided into real and imaginary parts:
 |
|
 |
(8.23) |
|---|
Plotting the real and imaginary parts as a
function of  , two straight
lines are obtained. Of these two, the real part intersects the vertical axis at
Rct and the imaginary part
at the origin. Both of the lines have a gradient of s. The plot is sometimes called the Randles plot.
, two straight
lines are obtained. Of these two, the real part intersects the vertical axis at
Rct and the imaginary part
at the origin. Both of the lines have a gradient of s. The plot is sometimes called the Randles plot.
If the impedance measurement is conducted off the equilibrium point, the measurement arrangement has to be such that a real stationary state can be reached (see comments at the beginning of Chapter 7.2). If the experiment is fast enough (< 1 min), we can compromise this requirement because the conditions during the experiment are nearly constant. Linearization is easier straight from the Butler-Volmer equation
 |
(8.24) |
|---|
which will, after linearization and using the Laplace transform, take the form
 |
(8.25) |
|---|
Quantities  represent the deviation from the surface
concentration caused by the AC input and are determined by the constant current
at each data point, see Equation (7.55);
represent the deviation from the surface
concentration caused by the AC input and are determined by the constant current
at each data point, see Equation (7.55);  and
and  are the deviations from constant current and
voltage, i.e. amplitudes of the AC signal.
are the deviations from constant current and
voltage, i.e. amplitudes of the AC signal.
Deviations from the surface concentrations are obtained as usual:
 ; ;  |
(8.26) |
|---|
We substitute Equation (8.26) in Equation (8.25) and place the current and voltage terms on different sides of the equation:
 |
(8.27) |
|---|
After little rearrangement, impedance is expressed as
 |
(8.28) |
|---|
Surface
concentrations of the stationary state  and
and
 can
naturally be determined from Equation (6.18), but if we want to see how the
charge transfer resistance and Warburg element change as a function of
potential, we will first sum up the surface concentrations using the general
equations (7.7) and (7.8):
can
naturally be determined from Equation (6.18), but if we want to see how the
charge transfer resistance and Warburg element change as a function of
potential, we will first sum up the surface concentrations using the general
equations (7.7) and (7.8):
 ; ;  |
(8.29) |
|---|
The surface concentrations can be determined
using Equation (8.24), taking into account that  and
and
 ;
;  is
defined in Equation (7.5).
is
defined in Equation (7.5).
 |
(8.30) |
 |
(8.31) |
|---|
Approximation, which eliminates the final term of the numerator, is based on practical experience: There is no point in running impedance measurements with high DC current densities because of the formation of convective currents. The term i/Fk0 is no more than 10-5 mol cm-3 in magnitude. It is possible to derive the results (8.30) and (8.31) directly from the assumption that the surface concentrations follow the Nernst equation; in fact, this assumption is typically made in AC voltammetry.
We can now write the charge transfer resistance and the potential dependency of Warburg element explicitly:
 |
(8.32) |
 |
(8.33) |
|---|
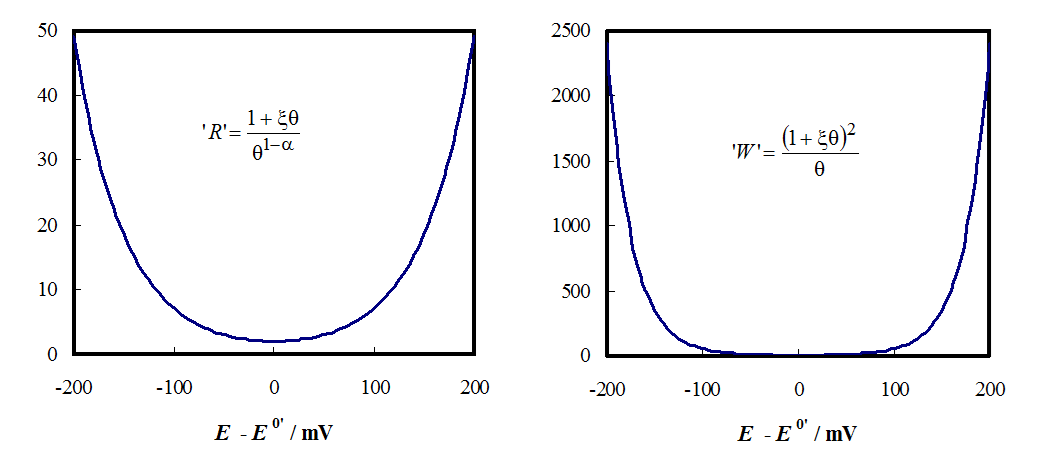
Figure 8.12. Potential dependency of charge transfer resistance (left) and Warburg
element (right) without their constants.  = 1
and n = 1;
= 1
and n = 1;  = exp[(nF)/(RT)(E
- E0’)].
= exp[(nF)/(RT)(E
- E0’)].
The value of the Warburg element becomes two orders of magnitude greater than that of the charge transfer resistance when the system is polarized, i.e. mass transfer to the electrode will be a limiting factor as shown in Figure 8.12. This is another reason why impedance measurements should not be conducted with high overpotential values.



