CHEM-E4106 - Electrochemistry D, Lecture, 9.1.2023-21.2.2023
This course space end date is set to 21.04.2023 Search Courses: CHEM-E4106
Kirja
6. Electrochemical reaction kinetics
6.7. Corrosion
In corrosion, two
simultaneous reactions are taking place on a metal surface so that the anodic
current is equal but opposite in sign to the cathodic current, Ia = |Ic| = Icorr,
the corrosion current. Although the net current in corrosion is zero the
exchange current can be quite high. Since the anodic and cathodic reactions
are not directly connected to one another, their potential dependence and charge
transfer coefficients ( ) are mutually independent.
The metal surface is settled at a corrosion potential Ecorr where the net current is zero. The corrosion
potential does not represent a true thermodynamic equilibrium because reactions
are proceeding, and it can also vary during the corrosion process as the
surface areas involved in the anodic and cathodic reactions change as corrosion progresses.
) are mutually independent.
The metal surface is settled at a corrosion potential Ecorr where the net current is zero. The corrosion
potential does not represent a true thermodynamic equilibrium because reactions
are proceeding, and it can also vary during the corrosion process as the
surface areas involved in the anodic and cathodic reactions change as corrosion progresses.
The dissolution of a metal can be written as an anodic reaction
M  Mn+ + ne- Mn+ + ne- |
(6.105) |
|---|
A cathodic reaction that
balances the electric charge depends on the pH of the solution. In a neutral or acidic
solution they are
O2
+ 4 H+ + 4
e–  2 H2O (neutral)
2 H2O (neutral) |
(6.106a) |
2 H+ + 2
e–  H2 (acidic)
H2 (acidic) |
(6.106b) |
|---|
In an alkaline solution, the following reactions are possible:
O2 + 2
H2O + 4
e–  4 OH–
4 OH– |
(6.107a) |
2
H2O + 2
e–  H2 + 2
OH–
H2 + 2
OH– |
(6.107b) |
|---|
Corrosion is often accompanied by the formation of an oxide film on the metal surface. Such a film is a poor charge carrier (semi-conductor) and passivates the surface, preventing further corrosion. Several metals such as various types of steel, chromium, aluminium, zirkonium, tantalum and titanium form a passivating film, the properties of which determine their corrosion resistance. Copper forms a stable Cu2O film or patina that gives the characteristic beautiful color to copper surfaces.
|
Table 6.1. Passivation potential of selected metals, EF0 (potential with the maksimum corrosion current); pH = 0, T = 298 K. (J.O’M. Bockris and A.K.N. Reddy, Modern Electrochemistry, Plenum Press, New York 1970, s. 1317.) |
|
|
Metal |
EF0 / V vs. NHE |
|
Au |
+1.36 |
|
Pt |
+0.91 |
|
Fe |
+0.58 |
|
Ag |
+0.40 |
|
Ni |
+0.36 |
|
Cr |
-0.22 |
|
Ti |
-0.24 |
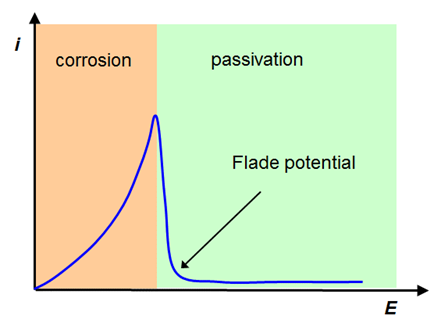
A great deal of corrosion studies concern iron (steel) due to its immense industrial significance. A qualitative current-voltage curve of steel in Figure 6.11 shows the passivation of the film and the termination of corrosion at potentials which are positive to the Flade potential.
The Flade potential is a
linear function of pH and often of the form
 |
(6.108) |
|---|
One of the oldest and
best known results about the corrosion of iron is that it dissolves readily in
diluted nitric acid but not in concentrated nitric acid. Iron dissolution is:
| Fe → Fe2+ + 2 e- | (6.109) |
|---|
The cathodic reaction is
the reduction of nitrate:
| NO3- + 2 H+ + 2 e- → NO2 + H2O | (6.110) |
|---|
Looking at reaction (6.110), the reaction rate should increase at a lower pH contrary to Equation (6.108). Obviously the corrosion of iron is determined by kinetics, and a purely thermodynamic analysis based on, for example, Bourbaix diagrams (Chapter 4) does not give the real picture. Based on Figure 6.11, it can be concluded that in very acidic conditions the corrosion potential of iron is on the negative side of the Flade potential and in moderately acidic solutions on its positive side. The impedance analysis of corrosion is discussed in Chapter 9.
