CHEM-E4106 - Electrochemistry D, Lecture, 10.1.2022-22.2.2022
This course space end date is set to 22.02.2022 Search Courses: CHEM-E4106
Kirja
6. Electrochemical reaction kinetics
6.8. Marcus theory
In 1992, Rudolf Marcus received the Nobel Prize in Chemistry for the theory of electron transfer that is commonly known as the Marcus theory. Its predecessors are the studies by Levich [1] and Dogonadze [2] about the reorganization of solvent molecules during a redox reaction. The approach taken by Marcus is based on statistical mechanics, considering the configurations of all molecules participating in the reaction. Each molecule is described as a harmonic oscillator, with an energy that depends in a parabolic manner on the deflection from its equilibrium position. As there are several molecules, each having several modes of vibration, a multi-dimensional energy space is obtained that is very difficult to visualize. We simplify the situation to the two-dimensional Figure 6.12. This approach [3] is closer to the models of Levich and Dogonadze, but is simpler than the accurate treatise by Marcus yet gives the same results.
On the right of Figure 6.11 the free energy profile of the oxidized species ’ox’ is seen. At configuration xox its free energy is Gox. Similarly, the reduced species’red’ has its energy profile on the left: its free energy at configuration xred is Gred. In this simple model, the reaction coordinate can be interpreted as the stretch of the molecular bond from its equilibrium length. The energy profiles intersect at configuration xc, where an electron is transferred from red to ox in an adiabatic process.
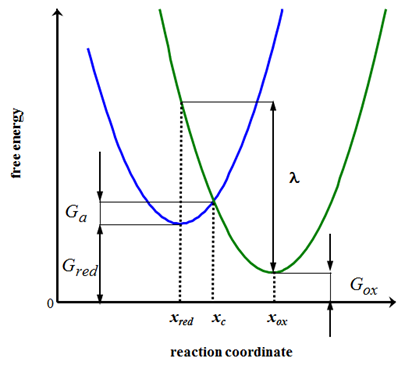
Let’s calculate the abscissa of the intersection of the parabolas, xc:
 |
(6.111) |
|---|
 ; ;  |
(6.112) |
|---|
Note that  . The factor
. The factor  is the energy of the fundamental vibration of
a harmonic oscillator that is obtained from its quantum mechanical solution; h is the Planck constant, 6.626×10–34
Js and
is the energy of the fundamental vibration of
a harmonic oscillator that is obtained from its quantum mechanical solution; h is the Planck constant, 6.626×10–34
Js and  is the vibration frequency (s–1). Inserting
the expression of xc into that of Gred, the height of the energy barrier (activation energy) is found to be
is the vibration frequency (s–1). Inserting
the expression of xc into that of Gred, the height of the energy barrier (activation energy) is found to be
 |
(6.113) |
|---|
 |
(6.114) |
|---|
The quantity  is
known as the reorganization energy, because
it represents the energy change when moving along either of the parabolas
between the points xred and xox, i.e. the change in the
molecular configuration without electron transfer. It is always a positive
quantity.
is
known as the reorganization energy, because
it represents the energy change when moving along either of the parabolas
between the points xred and xox, i.e. the change in the
molecular configuration without electron transfer. It is always a positive
quantity.
The rate constant of the reaction is expressed via the theory of activated complexes (absolute rate theory):
 |
(6.115) |
|---|
kB is the Boltzmann constant (1.38×10–23
J K–1) and  is what is known as transmission coefficient, which is unity for an adiabatic reaction. When the electrode
potential is changed, this changes
is what is known as transmission coefficient, which is unity for an adiabatic reaction. When the electrode
potential is changed, this changes  as discussed earlier in this chapter. The charge transfer coefficient
as discussed earlier in this chapter. The charge transfer coefficient  is therefore found to be
is therefore found to be
 |
(6.116) |
|---|
because
 |
(6.117) |
|---|
Thus, if  ,
,  , as is usually found to be the case in experiments in
the vicinity of an equilibrium potential. When
, as is usually found to be the case in experiments in
the vicinity of an equilibrium potential. When  is increased, the intercept xc approaches the point xred whence
is increased, the intercept xc approaches the point xred whence , Ga
= 0 and
, Ga
= 0 and  . When the intercept xc moves to the left side of xred, Ga starts to increase again, i.e. the reaction rate begins to
decrease. This range of potentials is known as the inverted region; its
existence was not verified until 1990s. If
. When the intercept xc moves to the left side of xred, Ga starts to increase again, i.e. the reaction rate begins to
decrease. This range of potentials is known as the inverted region; its
existence was not verified until 1990s. If  , i.e. the parabola of the species ox
is raised with respect to that of the species red, the activation energy
increases and
, i.e. the parabola of the species ox
is raised with respect to that of the species red, the activation energy
increases and  ultimately approaches unity (
ultimately approaches unity ( ).
).
The discussion above covers the reaction ’from
left to right’. Considering the inverse reaction ‘from right to left’, it is
seen from Figure 6.12 that the activation energy is higher by the amount of  . From Equation (6.113) we therefore see that
. From Equation (6.113) we therefore see that
 |
(6.118) |
|---|
We are now able to write down the reaction rate constants as the function of the electrode potential:
![\displaystyle k_{ox}=k'\text{exp}\left[-\frac{(\lambda-F(E-E^{0'}))^2}{4\lambda RT}\right] \displaystyle k_{ox}=k'\text{exp}\left[-\frac{(\lambda-F(E-E^{0'}))^2}{4\lambda RT}\right]](https://mycourses.aalto.fi/filter/tex/pix.php/ab1e6fab3744630f6992930c9f4f7d2b.gif) |
(6.119a) |
![\displaystyle k_{red}=k'\text{exp}\left[-\frac{(\lambda+F(E-E^{0'}))^2}{4\lambda RT}\right] \displaystyle k_{red}=k'\text{exp}\left[-\frac{(\lambda+F(E-E^{0'}))^2}{4\lambda RT}\right]](https://mycourses.aalto.fi/filter/tex/pix.php/208a12066887ff63cbfe3ad118b11740.gif) |
(6.119b) |
|---|
Equations (6.119) prove that when E – E0’ increases, the activation energy of oxidation decreases, in other words its reaction rate constant kox increases. Accordingly, the activation energy of reduction increases and kred decreases. It is also evident that at E = E0’
![\displaystyle k_{ox}=k_{red}=k'\text{exp}\left[-\frac{\lambda}{RT}\right] \displaystyle k_{ox}=k_{red}=k'\text{exp}\left[-\frac{\lambda}{RT}\right]](https://mycourses.aalto.fi/filter/tex/pix.php/b1013c62c51d8bdc0129430414f937d4.gif) |
(6.120) |
|---|
The reorganization
energy consist of the contributions from the reagents ( ) and the solvent (
) and the solvent ( ):
):  . The former includes
all the vibration modes of the molecules:
. The former includes
all the vibration modes of the molecules:
 |
(6.121) |
|---|
The solvent contribution  comes from the treatise by Marcus, but we
can be content with the final result:
comes from the treatise by Marcus, but we
can be content with the final result:
 |
(6.122) |
|---|
where r
is the molecular radius of the reagent and R
its distance to the electrode during the reaction;  and
and  are the optical and static relative permittivity of the solvent*, respectively, e is the elementary charge and
are the optical and static relative permittivity of the solvent*, respectively, e is the elementary charge and  the permittivity of
vacuum, 8.854
the permittivity of
vacuum, 8.854 10−12 F/m.
10−12 F/m.
Marcus theory has been further developed (Marcus-Hush, Marcus-Hush-Chidsey theories), but the merit of the original
Marcus theory is that it was able to explain the
potential dependence of the charge transfer coefficient  for example, for the first time. It also provides an estimate of the rate
constants in different solvents, as shown by Equation (6.122).
for example, for the first time. It also provides an estimate of the rate
constants in different solvents, as shown by Equation (6.122).
You can now test your conceptual knowledge by taking Quiz Chapter 6.
[1] V.G. Levich, Advan. Electrochem. Electrochem. Eng. 4 (1966) 249.
[2] R.R. Dogonadze, (Ed. N.S. Hush), Reactions of molecules at electrodes, Wiley 1971.
[3] Modified from: J. Albery, Electrode kinetics, Clarendon Press, Oxford, 1975.
* Relative permittivity is a
complex quantity that has limiting values  when
when  → ∞ and
→ ∞ and  when
when  → 0
where
→ 0
where  is the angular frequency of the dielectric measurement;
is the angular frequency of the dielectric measurement;  is the
permittivity that is also known as the dielectric constant (e.g. in water 78.4).
is the
permittivity that is also known as the dielectric constant (e.g. in water 78.4).
