CHEM-E6100 - Fundamentals of Chemical Thermodynamics, Lecture, 24.10.2022-7.12.2022
This course space end date is set to 07.12.2022 Search Courses: CHEM-E6100
You do not have the permission to view discussions in this forum
Topic outline
-
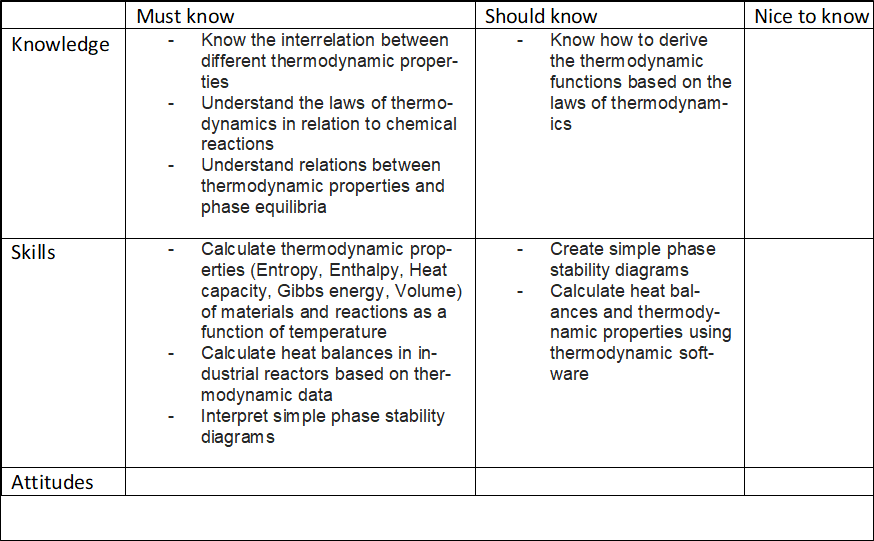
Compulsory course in Sustainable metals processing and Creative Sustainability (CHEM) majorsThermodynamics of pure substances and energetics of chemical reactions and their reactions with simple gas mixtures. Applications of the developed skills to the industrial applications including heat/energy balances.After the course the student will be able to•ILO 1 Know the meaning of and interrelation between different thermodynamic properties•ILO 2 Describe the laws of thermodynamics in relation to chemical reactions•ILO 3 Show the relations between thermodynamic properties and phase equilibria•ILO 4 Calculate thermodynamic properties (Entropy, Enthalpy, Heat capacity, Gibbs energy, Volume) of materials and reactions as a function of temperature•ILO 5 Solve heat balances in industrial reactors based on thermodynamic data in order to develop sustainable chemical and metallurgical processes•ILO 6 Interpret simple phase stability diagramsCore content analysis
Course textbook David R. Gaskell: Introduction to the thermodynamics of materials (2008) available in Aalto University library e-books in the ProQuest collection.
Lectures, exercises and workshops will be held on campusLectures twice a week 24.10-29.11.2022•Mondays 13:15-15:00 (Change: Exercise Wed 2.11 ↔ Lecture Mon 7.11)•Tuesdays 12:15-14:00•Lecture hall Platinium, C104, Critical Raw Materials Hub, Vuorimiehentie 2, except 31.10 and 28.11, Puunjalostustekniikka 1, 241 L1Exercises/tutorials starting 26.10.2022 ® 30.11.2022•Wednesdays 15:15-17:00 (Exercise November 2 will be moved to lecture time November 7)Workshops•11.11., 18.11., 25.11. and 2.12.•14:15-17:00
